Cultivation Techniques, news
Inhibition of Vibrio biofilm formation by ginger extracts
The in vitro inhibition of Vibrio biofilm formation and the protection of shrimp against AHPND when feed is supplemented with a ginger extract
As with most bacteria causing diseases in humans and animals, specific isolates of Vibrio parahaemolyticus bacteria are known to colonise and form biofilms on the chitin lining the stomach before releasing toxins that destroy the adjacent hepatopancreas. V. parahaemolyticus has been found as the cause of acute hepatopancreatic necrosis disease (AHPND) in penaeid shrimp. An ethanolic extract of ginger (Zingiber officenale) was found to inhibit the AHPND bacteria biofilms but not their growth in vitro.
When shrimp were fed feeds supplemented with extract of ginger, shrimp survival increased by 40-60% after infection with the AHPND bacteria when compared with the infected shrimp that were fed with normal un-supplemented feed. We suggest that biofilm inhibiting compounds, such as ginger extracts, can be used to treat or to reduce the negative effects of AHPND bacteria instead of the use of harmful antibiotics.
Ginger extracts contain bioactive compounds such as shogaol that was found to inhibit biofilm formation by AHPND-causing Vibrio bacteria
Bacterial biofilms and disease
Biofilm or surface-attached mode is the preferred mode of living of most bacteria. Biofilms are characterised by bacterial cells that are irreversibly attached to a substratum and embedded in a matrix of extracellular polymeric substances. Living in structured biofilm communities allows bacteria to proliferate more rapidly. It has been estimated by the US National Institute of Health that more than 80% of all human and animal bacterial diseases are associated with bacterial biofilms. Vibrio bacteria including the isolates that cause AHPND have also been shown to form biofilms in the chitinous cuticular lining of the shrimp forestomach before the bacterial cells produce and release PirA and PirB toxins that destroy the adjacent hepatopancreatic cells that eventually kill the affected shrimp, causing early mortalities.
To prevent or to treat AHPND, several strategies have been used, including the use of antibiotics which in itself is a very problematic practice. The most serious problem with antibiotic use is that it can lead to the development of antibiotic resistant bacteria which is currently the single most important global public health threat. Furthermore, antibiotic usage often leads to rejection or banning of antibiotic contaminated shrimp and shrimp products in the importing countries.
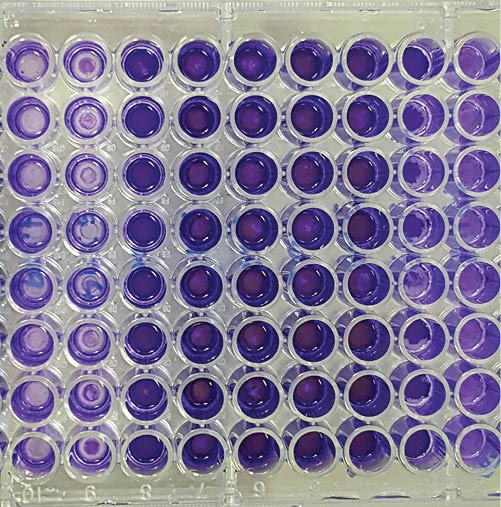
Microtiter plates containing wells with formed VAHPND biofilms that were stained with crystal violet and their thicknesses measured by absorbance readings.
To avoid antibiotic associated problems, we investigated the possibility of using extracts of plant materials that do not kill or inhibit the growth of the pathogenic bacteria but rather inhibit the bacteria biofilm formation in or on their hosts. These plant extracts were initially screened in vitro for their abilities to inhibit V. parahaemolyticus 3HP strain (VAHPND) biofilm formation in Mueller Hinton broth (MHB) with 1 v/v glycerol and 1.5% NaCl but not the bacteria planktonic cell growth. From our screening of several plant extracts, we found that the ethanolic extract of ginger was one of the extracts with such properties against VAHPND.
Inhibition of biofilm of AHPND bacteria but not bacterial growth
To prepare ethanolic extract of ginger, dry powder of ginger rhizomes (20g) was first extracted with 95% ethanol (300mL) using a Soxhlet’s apparatus set at 78°C for about 14h. The solvent (ethanol) was then removed from the extract obtained using a rotary evaporator set at 42°C resulting in a viscous yellowish brown material (2.74g). This constituted a yield of 137mg/g (13.7%) of ginger powder.
The concentrated extract obtained was diluted in dimethyl sulfoxide (DMSO) (40mg/mL) and diluted further with sterile distilled water to achieve final concentrations of 20mg/mL and 2mg/mL for use in Vibrio cell growth assays, biofilm inhibition assays and feeding experiments. These final diluted preparations (20μL/well) were added to the bacterial culture broth (180μL) of bacteria culture prepared from the overnight culture diluted with the same medium used to culture the bacteria which in this case, MHB medium supplemented with 1.5% sodium chloride and the diluted cells optical density (O.D. at 600 nm) was measured. The culture was diluted to until the O.D. of 0.01 was obtained in microtiter plates in aliquots to achieve final concentrations of 0.2mg ginger extract or 2mg ginger extract.
The biofilm cultures were incubated at 30°C without agitation overnight before the formed biofilms were stained with crystal violet and their thicknesses were measured by absorbance readings at O.D. 600 nm. The higher the reading, the thicker the formed biofilms. A similar experimental set up was also prepared to determine if the extract inhibits VAHPND planktonic cell growth. The bacteria were cultured in uncoated plate using MHB medium with 1.5% NaCl and incubated overnight at 30°C with agitation at 200rpm.
Light absorption at 600nm was used as the cell growth indicator. It was observed that the ginger extract at both of these concentrations were found to significantly inhibit VAHPND biofilm but not the growth of the bacteria so the ginger extract at lower concentrations of 400μg/mL, 300μg/mL, 200μg/mL and 100μg/mL were also tested and all concentrations were found to inhibit the bacteria biofilm formation (Figure 1). For more detailed information on protocols used and results, please refer to Soowannayan et al. (2019) published in Aquaculture 504, pp. 139-147.
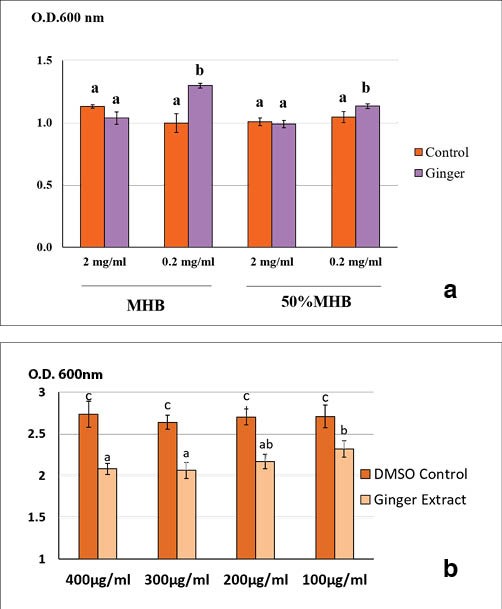
Figure 1. Average optical density readings at 600nm indicative of Vibrio parahaemolyticus 3HP planktonic cell growth or growth in broth (a) and biofilm thickness (b). In figure (a), the planktonic cells of the bacteria were grown in half and full-strength MHB medium with and without ginger extract at two different concentrations (2mg/mL and 0.2mg/mL). The extract at the lower concentration (0.2mg/mL) did not inhibit bacterial growth but significantly promoted it when compared to controls. In figure (b), average O.D. reading of crystal violet stained biofilms in the presence and absence of ginger extract at different concentrations and in MHB. The extract significantly (P<0.05) inhibited biofilm at all extract concentrations when compared with the controls. Adapted from Soowannayan et al. (2019).
Supplemented feed reduced shrimp mortality after VAHPND infection
The ginger extract was then mixed with a commercial post larvae shrimp feed (at two different concentrations; 0.2mg/g and 2mg/g feed) and fed to triplicate groups of whiteleg shrimp, Penaeus vannamei for 7 days before the shrimp were challenged by immersion method with VAHPND at 105CFU/mL. We continued to feed shrimp with their respective feed while the control shrimp were fed with normal un-supplemented feed for a further 9 days, during which their health and mortalities were observed and compared.
From the results obtained, ginger supplemented feed at 2mg/g or 0.2mg/g concentrations were found to protect whiteleg shrimp against VAHPND infection. The average survival of the ginger extract fed shrimp were found to be 50.0% and 42.5% respectively which are 42.5% and 35 % higher than those of the infected shrimp that were fed un-supplemented feed (7.5% survival). The survival of the unchallenged control group was 92.5% (Figure 2). Histopathological examination of hematoxylin and eosin (H&E)- stained tissues of AHPND isolate 3HP-challenged shrimp showed less severe AHPND pathologies in infected shrimp that were fed with ginger extract supplemented feeds (Figure 3).
In this study we also found that there was no apparent negative effect of the ginger extract on feed palatability or on shrimp growth. Three potentially bioactive compounds known to occur in ginger extracts (6-gingerol, 8-gingerol, and 6-shogaol) were also tested for efficacy in biofilm inhibition in AHPND, and 6-shogaol was found to be the most potent. None of these bioactive compounds were found to affect bacterial growth in broth. The results of this study suggested that the use of gingerbased or other feed additives that inhibit biofilm formation may constitute a practical approach to reduce the negative impact of AHPND in shrimp aquaculture.
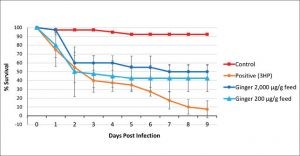
Figure 2. Graph showing cumulative % survival of Penaeus vannamei post larvae fed with ginger extract-supplemented feed after challenge with AHPND-causing Vibrio bacteria (3HP strain). Adapted from Soowannayan et al. (2019).
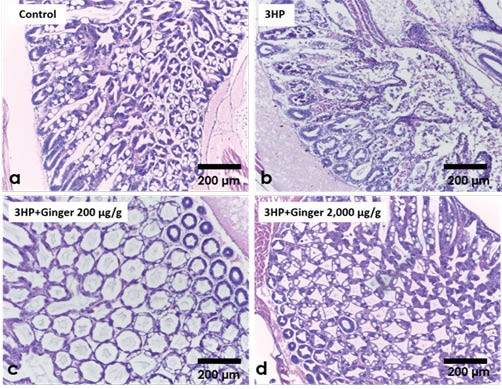
Figure 3. (a) Hepatopancreatic tissue of an unchallenged negative control shrimp showing normal histology; (b) Hepatopancreas of a moribund shrimp from the VAHPND 3HP-challenged group fed un-supplemented feed and showing massive sloughing of tubule epithelial cells pathognomonic for AHPND; (c) Hepatopancreas of a moribund shrimp from the 3HP-challenged group fed with 200μg ginger supplement and showing abnormal collapsed tubule epithelia but no pathognomonic AHPND lesions; (d) Hepatopancreas of a surviving shrimp from the 3HP-challenged group fed with 2000μg ginger supplement and showing normal histology. Adapted from Soowannayan et al. (2019).
By Aqua Culture Asia Pacific
Reference: https://aquaasiapac.com/2020/10/02/inhibition-of-vibrio-biofilm-formation-by-ginger-extracts/
See more:

 Tiếng Việt
Tiếng Việt