Cultivation Techniques, news
Effects of cold stress on hemolymph characteristics of Pacific white shrimp
Findings improve knowledge of the molecular mechanisms in the hemolymph

Authors used physiological, transcriptomic, and metabolomic analyses to investigate the response of Pacific white shrimp to cold stress. The results indicated that cold stress disrupted various physiological processes and that shrimp exhibited response mechanisms to enhance cold tolerance, regulating changes in key genes, and in amino acid and lipid metabolism to increase energy supply and protect cell membrane stability. This research provides some references regarding the molecular mechanisms involved in responding to cold stress and potential strategies to improve cold tolerance in P. vannamei. Photo by Francisco Miranda.
Water temperature changes are common stressors in aquaculture; among these stressors, low temperature is a serious environmental stress factor. In shrimp culture, low temperatures can affect shrimp health by suppressing the immune system and disrupting physiological processes.
For the Pacific white shrimp (Penaeus vannamei), it has been reported that it can grow normally between 25 and 35 degrees-C, but will stop feeding at temperatures lower than 18 degrees-C and will die below 12 degrees-C.
The molecular mechanisms underlying the coregulation of genes and metabolites in the cold tolerance of P. vannamei remain unclear. To the best of our knowledge, there is still a lack of research through an integrated analysis of physiological (study of functions and mechanisms in a living organism), transcriptomic (techniques used to study an organism’s transcriptome, the sum of all of its RNA transcripts which express the information content of an organism as recorded in the DNA of its genome), and metabolomic (study of chemical processes involving metabolites, the products of cell metabolism) studies on the response mechanism to cold stress of P. vannamei. Therefore, it is of great significance to systematically understand the molecular mechanisms based on the hemolymph (blood analog in invertebrates) of P. vannamei in response to cold stress.
This article – condensed from the original publication (Zhu, J. et al. 2024. Effects of Cold Stress on the Hemolymph of the Pacific White Shrimp Penaeus vannamei. Fishes 2024, 9(1), 36) – discusses the results of a study of the responses of P. vannamei to cold stress using physiological, transcriptomic and metabolomic analyses.
Study setup
The study was conducted at the National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University (Shanghai, China). Healthy P. vannamei (weight: 9.06 ± 0.23 grams, length: 10.0 ± 0.4 cm) were obtained from the Institute of Oceanology and Marine Fisheries, Jiangsu, Nantong, China. Shrimp were temporarily cultured in an indoor tank for seven days before the experiment.
A total of 300 shrimp were randomly selected and divided into three 1,000-liter PVC tanks as three replicates, and all shrimp were adapted to the environment for two days before the experiment. The starting temperature of the experiment was set at 24 degrees-C (control group, CK) and was automatically cooled by a chiller at a rate of 2 degrees-C every two hours. Then, the cooling was stopped when the water temperature reached the predetermined temperature (22, 20, 18, 16, 14 and 12 degrees-C) and remained stable for 22 hours. During this period, these temperature points (24 degrees-C /CK, 20 degrees-C /T1, 18 degrees-C/T2, 14 degrees-C/T3 and 12 degrees-C/T4) were set as sampling points. Shrimp were randomly selected from each temperature point and quickly anesthetized on ice (10–15 sec).
Hemolymph samples were collected from shrimp at each point (CK, T1, T2, T3, T4) and then immediately processed for physiological analysis]. The experimental process is shown in Fig. 1. For detailed information on the experimental design, animal husbandry, and sample collection and analyses, refer to the original publication.
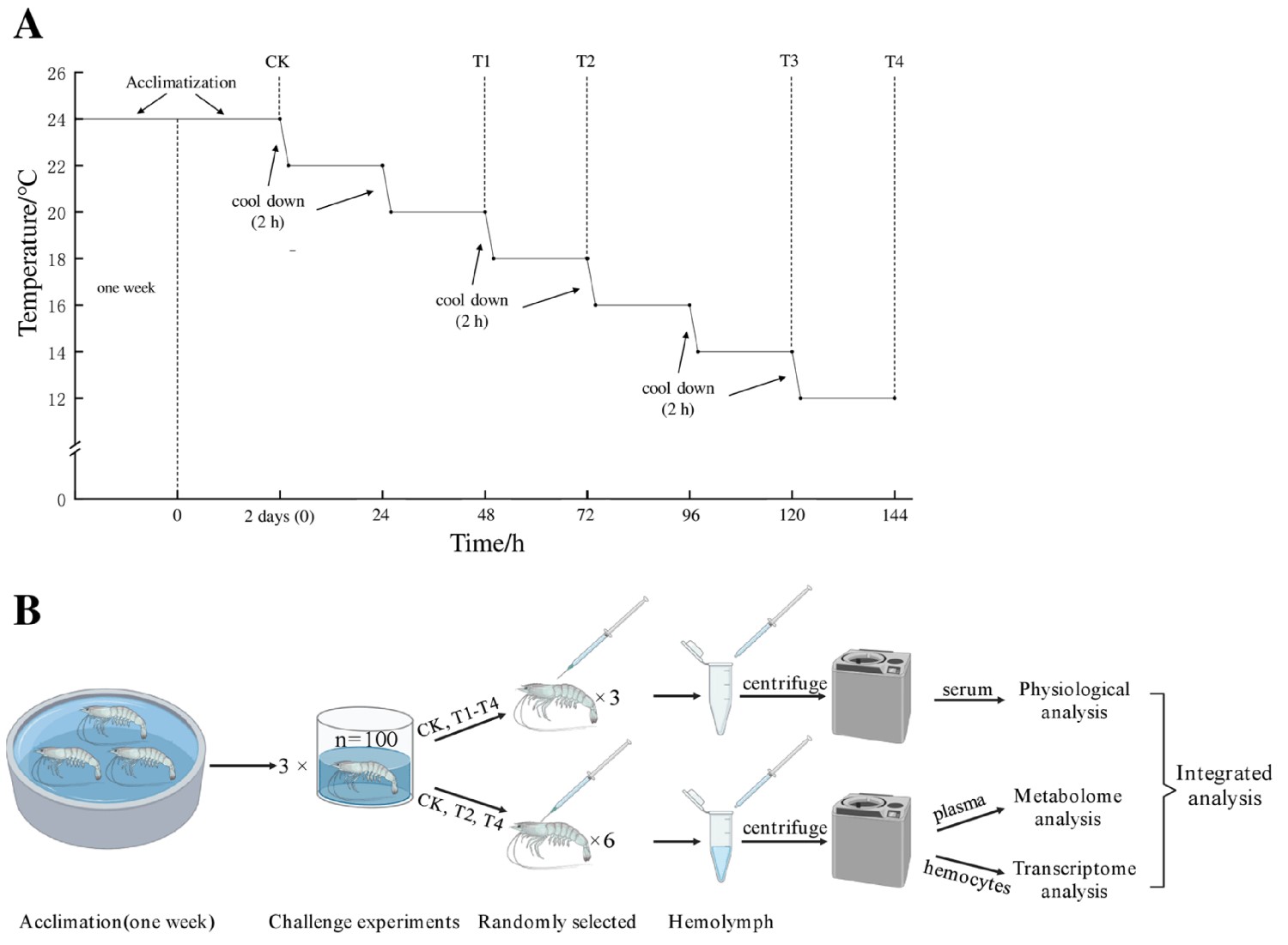
Fig. 1: Experimental process. Control check (CK/24 degrees-C), treatment group 1 (T1/20 degrees-C), treatment group 2 (T2/18 degrees-C), treatment group 3 (T3/14 degrees-C), treatment group 4 (T4/12 degrees-C). (A): After acclimating, the starting temperature of the experiment was 24 degrees-C, and samples collected at this temperature were named as CK (control group) before cooling. The temperature of each drum was automatically cooled by a chiller at a rate of 2 degrees-C /2 h, the cooling was stopped when the water temperature reached the predetermined temperature (22, 20, 18, 16, 14, and 12 degrees-C) and remained stable for 22 h, and then sampling was performed. (B): After centrifuging, serum from three shrimp in each drum were collected at CK, T1, T2, T3, and T4 for physiological analysis, and hemocytes and plasma from six shrimp in each drum were collected at CK, T2 and T4 for omics analysis.
Results and discussion
Overall, the results of this research indicated that cold stress can cause significant changes in enzyme activity, gene expression and metabolic product levels in P. vannamei (Fig. 2).
In crustaceans, hemolymph serves important functions, including transporting nutrients, metabolic waste, hormones and neuropeptides, while also acting as an important mediator of the immune response; the regulation of hemolymph homeostasis (steady state) is critical to the health of animals. Changes in metabolites under low-temperature conditions can be influenced by changes in genes and vice versa.
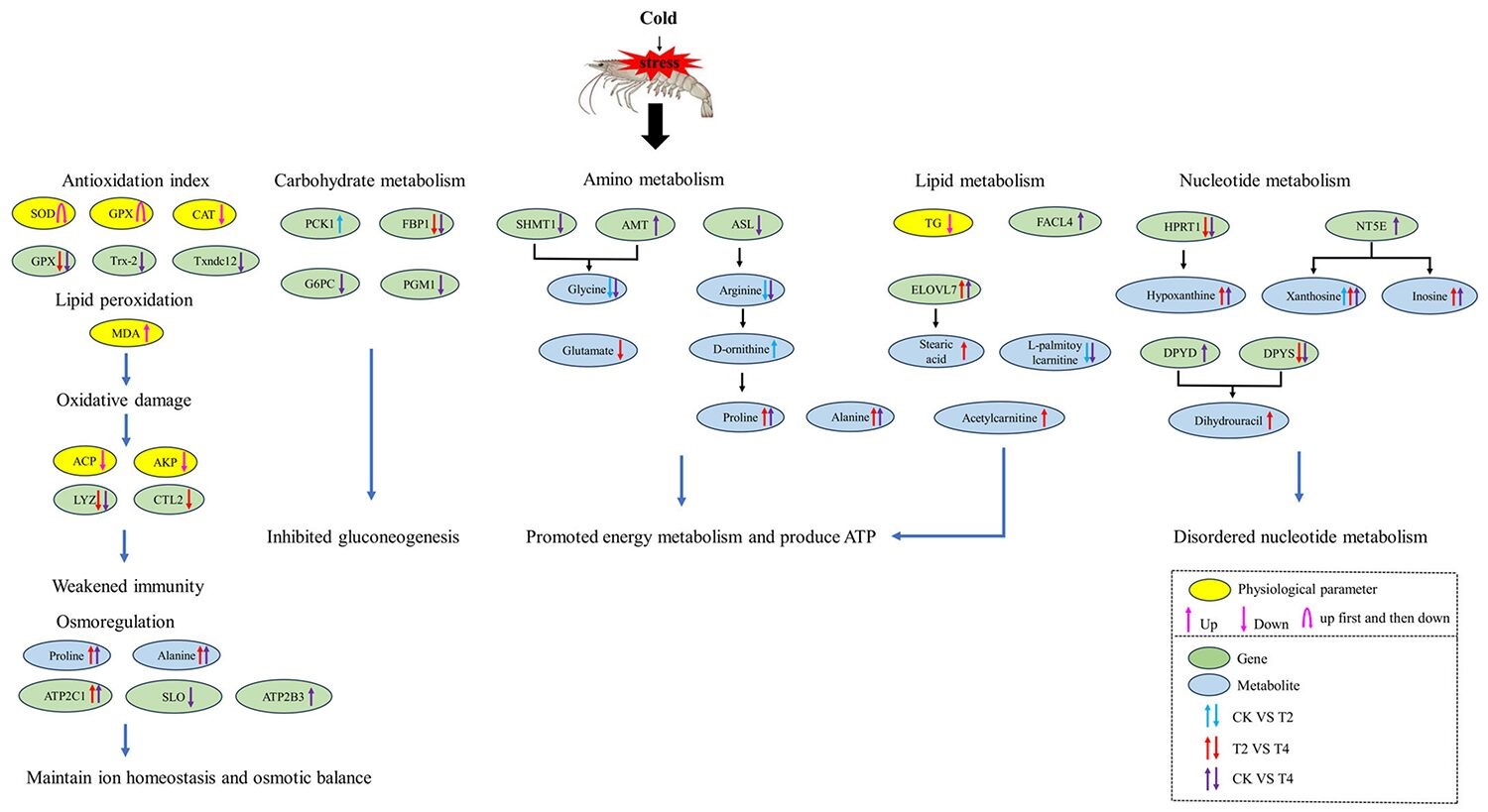
Fig. 2: The potential regulatory mechanisms of P. vannamei under cold stress. CK (24 degrees-C), T2 (18 degrees-C) and T4 (12 degrees-C). Adapted from the original.
Upregulation (the process by which a cell increases the production and quantities of its cellular components, such as ribonucleic acid, RNA and proteins, in response to an external stimulus) or downregulation (complementary process to upregulation) of certain genes under low-temperature conditions may lead to the activation or inhibition of related metabolic pathways, resulting in an increase or decrease in metabolite levels. Furthermore, changes in metabolites can regulate gene expression through feedback mechanisms. For example, the upregulation of certain metabolites under low-temperature conditions induced the expression of related genes by activating certain physiological processes.
Under long-term cold stress, the metabolic response of the organism may shift toward conserving energy and prioritizing survival. Based on the transcriptome results, we observed that the gluconeogenesis (metabolic pathway that results in the biosynthesis of glucose from certain non-carbohydrate carbon substrates) metabolism pathway was significantly downregulated in hemocytes under cold stress. In our study, the expression of two key enzymes involved in gluconeogenesis were downregulated under cold stress, indicating that their activity was reduced, leading to reduced glucose synthesis.
Under cold stress, the metabolism of some amino acids may be altered. Metabolomic data revealed significant changes in the levels of many metabolites involved in amino acid metabolic pathways under cold stress. Our results indicated that cold stress has a significant impact on the amino acid metabolism of shrimp; when carbohydrate utilization is hindered, it shifts toward using amino acids as an energy source. Additionally, there was a decrease in amino acids associated with growth and other specific functions. This metabolic response may affect the overall health and physiological processes of shrimp, including growth, development, and immune function. In addition, some other amino acids, such as alanine, proline, D-ornithine, and L-glutamine, were significantly increased when the temperature decreased in our study.
The accumulation of the amino acids proline and alanine under cold stress seems to be a common feature of invertebrates. Proline has a protective effect against cold stress in abiotic stress, and the accumulation of proline contributes to the cold tolerance and low-temperature adaptation of organisms. Proline can provide important energy reserves for cold tolerance and cold acclimation, and the oxidation of proline to alanine can theoretically provide metabolic energy. Previous studies have found that cold adaptation as well as cold tolerance in crustaceans are associated with a significant increase in proline levels.
In our experiment, the decrease in arginine levels was accompanied by a significant increase in the concentrations of other amino acids, indicating that some amino acids were broken down to produce more proline under cold stress, which may improve the tolerance of the organism to low temperature through mechanisms such as osmotic protection, antioxidant defense and energy metabolism. This may be one of the important mechanisms by which organisms improve cold resistance.
Our data also showed that the accumulation of amino acids such as proline, alanine and others under cold stress contributed to the enhanced low-temperature tolerance of P. vannamei through mechanisms including osmotic protection, antioxidant defense, energy metabolism and reduction in oxidative stress damage. These results further revealed that amino acids are the main energy resource in the response of P. vannamei to cold stress.
On the effect of cold stress on lipid metabolism, lipids are one of the energy sources that are easiest to obtain, with triglycerides (TG) decomposing into glycerol and fatty acids to provide energy. We observed that TG levels in the serum of P. vannamei decreased significantly during low-temperature stress, indicating that TG was consumed to provide energy. Our data indicated that P. vannamei adjusted lipid metabolism in response to cold stress, increasing energy production to sustain cellular energy homeostasis under low-temperature stress.
Regarding cold stress-induced alterations to nucleotide (the basic building block of nucleic acids, RNA and DNA) metabolism, overall, the changes we documented in our study indicated that cold stress could lead to disordered nucleotide metabolism, which may have negative effects on energy metabolism, signal transduction (the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events) and nucleotide synthesis ability.
In low-temperature environments, the metabolic rate of P. vannamei will decrease, which means that their immune system may not function as efficiently as at normal temperatures. The immune defense system can be activated by oxidative stress in aquatic animals. The enzymes AKP and ACP play important roles in the immune system, and AKP and ACP in crustaceans participate in the first line of nonspecific immunity. Our results showed that AKP and ACP activities decreased significantly after low-temperature stress, indicating that cold stress damaged the nonspecific immune barrier of the organism and compromised the immune system of P. vannamei.
Transcriptome analysis showed that the expression of certain genes was downregulated in P. vannamei under low-temperature stress. Under cold stress, the downregulation of these genes in P. vannamei weakened its immune recognition and defense system. In summary, cold stress caused stress responses and metabolic disorders in P. vannamei, disrupting the antioxidant system and leading to immune suppression.
The results of this study provide some important insights into the molecular mechanisms of cold adaptation in P. vannamei and potential strategies to increase its cold tolerance, which are important references for studying the cold stress response of shrimp.
Perspectives
This study employed an integrated multi-omics approach, combining physiology, transcriptome and metabolome data, to explore the regulatory mechanisms involved in the response of P. vannamei to cold stress. The results indicated that cold stress can cause significant changes in enzyme activity, gene expression and metabolic product levels in P. vannamei.
Due to the inhibition of carbohydrate metabolism, organisms accelerate the consumption of certain amino acids, such as glycine and glutamate, to obtain more energy to maintain their energy metabolism. In addition, the accumulation of some amino acids, such as proline, alanine and L-glutamine, may improve cold tolerance through mechanisms such as osmotic protection, antioxidant defense and energy metabolism.
When the temperature dropped to the tolerance limit of P. vannamei, metabolic homeostasis and physiological balance were disrupted. The antioxidant defense was initially activated but became overwhelmed at extremely low temperatures, ultimately leading to oxidative damage.
Additionally, immune levels were found to be severely suppressed under cold conditions. These findings could contribute to a better comprehension of the molecular mechanism underlying hemolymph in the P. vannamei response to cold stress and provide some important references to study the response to cold stress in shrimp.
Follow by Dr. Xihe Wan

 Tiếng Việt
Tiếng Việt