Cultivation Techniques, news
Effect of dietary Garlic extract on growth, feeding parameters, hematological indices and body composition of Litopenaeus vannamei
Abstract
In this study, Effects of garlic (Allium sativum) extract on growth indices and hemolymph parameters of Litopenaeus vannamei with mean weight 18.21±0.29 g were studied for a period of 56 days. This study was conducted inside 300–liter polyethylene containers as four treatments each with four replicates and each replicate testing 5 shrimps. A basic diet supplemented by 0 (control), 40, 80 and 100 ppm of garlic extract was provided for shrimps. Shrimp biometry was performed at the end of experiment and hemolymph was collected by 2ml syringe. The results showed that garlic extract positively affected daily feed intake, feed conversion efficiency, protein efficiency ratio, molting rate (P<0.05), but no significant change in weight gain rate, specific growth rate, condition factor and hepatosomatic index (P>0.05). Also, treated showed significant changes in body protein (increased levels), fat and ash composition (decreased levels) compared with control group (P<0.05). Total hemocytes count was not affected but differential hemocytes count and total plasma protein was significantly changed treated shrimps compared with control shrimps (P<0.05). Based on the results obtained from this study, garlic extract may be applied as a food supplementation in shrimp aquaculture.
Introduction
The application of immunostimulants in shrimp aquaculture is increasingly gaining interest as an environmentally safe alternative to antibiotics and chemotherapeutics (Song et al., 1997). Shrimp possess an innate immune system, consisting of cellular and humoral elements. Hemocytes play a central role in the non-specific immune response of shrimp, which rely mainly on phagocytosis, melanization, encapsulation, cytotoxicity, and clotting (Sritunyalucksana et al., 1999). Humoral defense factors, such as clotting proteins, agglutinins, hydrolytic enzymes, and antimicrobial peptides are released upon lysis of hemocytes, which is induced by lipopolysaccharides (LPS), peptidoglycans, and β 1,3-glucans (Chisholm and Smith, 1995; Muta and Iwanaga 1996; Destoumieux et al., 2000).
Diets containing immunostimulants are used in aquaculture in order to increase resistance to stress and diseases of cultured fishes and invertebrates by alerting the immune system (Rendon and Balcazar, 2003; Donate et al., 2010).
Aquaculture represents one of the main food providers in the world. In shrimp farming, Whiteleg shrimp (Litopenaeus vannamei) is the primary penaeid shrimp currently being cultured in Central and South America (Burge et al., 2007; Chang-Che and Jiann-Chu, 2008). However, during the past two decades, worldwide commercial shrimp farming has suffered outbreaks of diseases caused mainly by Vibrio bacteria and viruses due to a deteriorated pond environment (Lo et al., 2003).
The white shrimp, Litopenaeus vannamei , which is naturally distributed along the Pacific coasts of Central and South America has become the most important cultured species worldwide due to successful development of techniques for manipulating brood stocks, spawning and seed production (Tayag et al., 2010).
Garlic (Allium sativum) is a perennial bulb forming plant that belongs to the genus Allium and family Liliaceae, which has been used for centuries as a flavoring agent, traditional medicine and a functional food to enhance physical and mental health. Garlic effects have been studied in different forms of extracts: aqueous, ethanol and dried powder in 16 month-old rats (Shin and Kim, 2004). It contains a variety of organosulfur compounds, such as allicin, ajoene, S-allylcysteine, diallyl disulfide, S methylcysteine sulfoxide and S-allylcysteine (Chi et al., 1982). Studies on garlic as an alternative to growth promoters in livestock production were conducted and its beneficial effects on growth, digestibility and carcass traits have been reported (Bampids et al., 2005; Tatara et al., 2008). Dietary garlic as a growth promoter in Nile tilapia (Oreochromis niloticus) improved body weight gain, feed intake and feed efficiency (Diab et al., 2002; Shalaby et al., 2006). Also, garlic has potential role as prophylactic and therapeutic agent in aquaculture (Amagase et al., 2001). Garlic can be used to control the pathogens, especially bacteria and fungi and it increases the welfare of fish (Corzo-Martinez et al., 2007). Expression of innate immunity genes in kuruma shrimp Marsupenaeus japonicus was studied after in vivo stimulation with garlic extract (allicin) by Tanekhy and Fall (2015). Also, efficacy of garlic was studied on the survival, growth and haematology of Penaeus monodon post larvae (Vidhya Malar and Maria Charles, 2013). Labrador et al. (2016) observed that the white leg shrimp fed the diet containing 6% garlic powder obtained the highest weight gain. Shrimps being fed by Artemia enriched with 200mg garlic extract per kg food showed the best growth, survival rates and length (Javadzadeh et al., 2012).
The aim of this study was to examine the effects of the dietary Garlic extract as a feed additive on growth performance, feed utilization, biochemical parameters of the body and some haematological indices in Pacific white shrimp, Litopenaeus vannamei.
Material and Methods
Preparation of garlic extract and the experimental diets.
Fresh garlic was purchased from the local market in Hamadan (Iran). Peeled fresh garlic was chopped into small pieces, dried and ground finely and then macerated in 95% ethyl alcohol incubated at room temperature and after three days, filtered through Whatman filter paper ( 42 micron) (Zargari, 1997). The procedure was repeated three times to ensure exhaustive extraction of the plant material. The extract was pooled together, concentrated and the solvent removed by evaporation under reduced pressure in a rotary evaporator at 75ºC. The garlic extract was kept in a refrigerator at 4°C before use. Experimental diets were prepared by combining powdered commercial shrimp pellet (21-beiza company, Iran) with plant extract and 2% gelatin to produce four diets: (1) commercial diet as control group, (2) 40 mg/kg of food, (3) 80 mg/kg of food, (4) 100 mg/kg of food. After drying, the pellets were stored in plastic packets at 4 °C until being used. Composition of the basic diet used for white shrimp, Litopenaeus vannamei in this study included of 33% Crude protein, 8% Crude lipid, 4% Crude carbohydrate,14% ash and 10% moisture.
Shrimp rearing
Ninety clinically healthy Pacific white shrimps (Litopenaeus vannamei Boone) were obtained from a commercial farm in Bushehr, South of Iran. Upon arrival, they were acclimated to laboratory conditions for 15 days in fiber glass-reinforced plastic (FRP) tanks and fed by the commercial diet. The laboratory conditions during the acclimation period were daily monitored and maintained at the same level. There were four treatments in four replicates. Each replicate consisted of 5 shrimps with mean weight 18.21±0.29 g in a 300 liter circular FRP tank with 200 liter of water at a salinity of 29.42± 0.64 ppt. Aeration was supplied by a single air-stone to maintain the dissolved oxygen at 7.72 ± 0.18 mg l-1. Shrimp were fed thrice daily at 08:00, 11.00 and 18.00. Molts, feces and dead shrimps were daily removed and 30% of water in each tank was exchanged by new sea water (Javadzadeh et al., 2012). During the experimental period, the water temperature was maintained at 25.19 ± 0.087 °C and pH 8.02 ± 0.051.
Growth and nutritional parameter
At the end of the feeding trial, total weight and length of shrimp were measured and hepatopancreas was collected. The relative weight gain rate (WG%) = 100× (Wt – W0)/ W0 ×100; Molting rate (MR, %.day-1)= (Nm / Ns) / T, Specific growth rate (SGR) = ln Wt-ln W0 ÷ T × 100, feed intake (FId)= 100 × F/(T×(Wt+ W0)/2), food conversion efficiency (FCEd)= 100× (Wt- W0)/F , Protein Efficiency Ratio (PER)= (Wt- W0)/P , hepatosomatic index (HIS) = Liver weight/ fish body weight, Condition Factor (CF%)= (Wt× 100)/ (L)3 and daily feeding rate (DFR, %) = feed intake/((W0+Wt)/2 days of the experiment)×100 were calculated where, Wt and W0 are the final and initial wet body weight of the shrimp, respectively; Nm, molting frequency; Ns, the number of shrimps per aquarium, respectively; T, the time of the experiment lasted; F, the total food consumed in dry weight; P, the total Protein consumed in dry weight; L, the total length of each shrimp (Wang et al., 2004).
Hematological Parameters (Total Haemocyte count (THC) and differential Haemocyte count (DHC))
Half a ml of haemolymph was collected from second swimming leg by using a 21-gauge hypodermic needle and by 2-ml syringe. Each syringe was pre-filled with 0.3 ml of anticoagulant (10 mM Tris–HCl, 250 mM sucrose, 100 mM sodium citrate, pH 7.6). Anticoagulant was added to make an equal volume ratio of hemolymph to anticoagulant. A volume of 50 ml anticoagulated hemolymph was fixed with an equal volume of neutral buffered formalin (10%) for 30 min to measure the total haemocyte count (THC) and differential hemocyte count (DHC). The remaining anticoagulated hemolymph was centrifuged at 300×g for 10 min at 4°C to separate the hemocytes from plasma. The plasma total protein was measured by photometric method by using trading diagnostic kit (Thomas, 1998).
Total hemocytes were counted using a hemocytometer (Boeco, Germany) and light microscope at 100×. Fixed hemolymph was smeared on a slide and stained with Giemsa solution (10%) for 10 min. The differential hemocytes includeHyaline, Semi-granular and Granular cells were characterized according to Tsing et al. (1989) and 100 cells from each smear were counted under a light microscope.
Body biochemical composition analysis
The experimental diets were sampled before adding and after the Garlic extract for analysis of crude protein, fat and ash. At the end of the trial, shrimp per each tank were randomly sampled and meat yields (%) were evaluated after peeling the shell and removing the head. The meat was homogenized for the analysis of moisture, crude protein and crude ash. The chemical composition was determined following procedures (AOAC, 1984): crude protein (Nx6.25) by the Kjeldahl method (Kjeltec 2100 Distillation Unit, Foss Tecator, Hoganas, Sweden), crude fat using an ether extraction method, moisture by oven drying at 105[degrees]C for 24 h, ash using a muffle furnace at 550[degrees]C for 4 h.
Statistical Analysis
Results were analyzed using SPSS (version 16.0) software. Data was normalized by Kolmogorov Smirnov Test. One way ANOVA and Tukey’s multiple range tests were used to determine the significance of differences between groups. All the results were expressed as means ± standard error (M ± SE) and significant differences were expressed at a significance level of P<0.05.
Results
Growth parameter
At the end of the 56 days feeding trial, molting rate was high in shrimp fed by 80 and 100 mg kg-1 Garlic extract and lowest in shrimp fed by diet 40 mg kg-1 and group fed by the diet without Garlic extract supplementation (p < 0.05) (Table 1).
There were no significant increase in the weight gain rate, SGR, HIS and CF among treatments but the results presented in Table 1, indicated a trend of increased weight gain rate and SGR in group 80 mg kg-1garlic extract supplementation. Also, the shrimp fed by 100 mg kg-1garlic extract had higher HIS than shrimp fed by other concentrations of garlic extract and the shrimp fed by 40 mg kg-1garlic extract had higher CF than shrimp fed by other concentrations.
Table 1: Growth parameters and feeding rate of white leg shrimp fed by different experimental diets supplemented by different level of garlic extract.
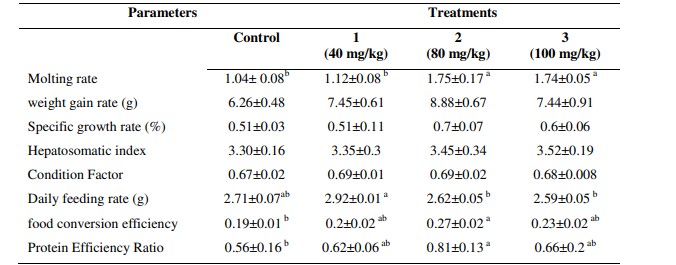
* Different letters show significant differences between treatments (p<0.05). All data were presented in Mean ±SE.
Feeding parameter
At the end of the 56 days feeding trial, DFI was high in shrimps fed by 40 mg kg -1garlic extract and lowest rate for shrimp fed by the diet with 100 mg kg -1 garlic extract supplementation (p < 0.05). The Feed conversion efficiency in Group 80 mg kg-1garlic extract was significantly higher than shrimp fed by other concentrations. The PER followed the same pattern as the Feed conversion efficiency (Table 1).
Hematological Related Parameters
The THCs and DHCs of shrimp fed by diets containing garlic extract and control diet for 56 days are shown in Fig. 1. No significant differences in the THCs of shrimp were recorded and the THCs of shrimp fed by control and garlic extract diets including of 40, 80 and 100 mg kg-1 garlic extract were (8.75 ± 1.96) ×103, (8957.7±1.36) ×103, (13431.1±2.68) ×103, and (15005.7± 4.01) ×103cells ml_1, respectively (Fig. 1A). Also, the Granular Cells were the most affected cell types by garlic extract treatments, with the highest value in the group fed by 100 mg kg -1 at 56 days from the start of feeding (Fig, 1B). The hyaline cell in Group 80 mg kg-1garlic extract was significantly lower than shrimp fed by 40 mg kg -1garlic extract and control diet. In addition, semi-granular cell in Group 80 mg kg-1garlic extract was significantly lower than shrimp fed by other diets. Moreover, the shrimp fed by 100 mg kg-1garlic extract had higher total plasma protein than shrimp fed by other diets.
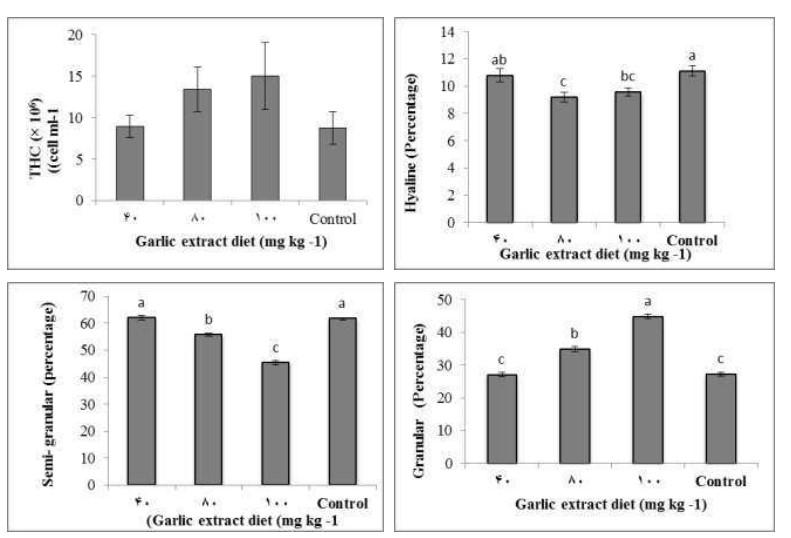
Fig. 1: Total hemocyte counts (A) , Hyaline cell (B) Semi-granular cell (C) and Granular cell (D) of Pacific white shrimp, Litopenaeus vannamei, fed by diet without Garlic extract as the control and diets with different concentrations of Garlic extract of 40, 80 and 100 (mg kg diet)_1 for 56 days. Data (mean ±SE) with different letters significantly differ (p < 0.05) among treatments.
Body composition Analysis
The results of body composition analysis including total protein, fat, ash and moisture from muscle tissues samples are given in Table 2.
The significant highest protein content of 18.92 ± 0.47 % in muscle tissue was observed in the shrimp fed by 80 mg kg-1garlic extract than shrimp fed by other diets. Lipid content in muscle tissue were found to be significantly lowered for shrimp fed by 40, 80 and 100 mg kg -1 garlic extract and highest for shrimp fed by diet without garlic extract supplementation (p < 0.05). Additionally, the ash content in muscle tissue were determined to be significantly increased in 40, 80 and 100 mg kg -1 garlic extract and highest for shrimp fed by diet without garlic extract supplementation (p < 0.05).
Table 2: Body biochemical composition of white leg shrimp fed by different experimental diets supplemented by different level of garlic extract
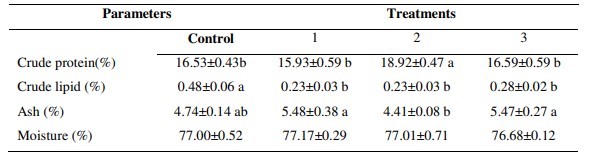
* Different letters show significant differences between treatments (p<0.05). All data were presented in Mean ±SE.
Discussion
Garlic (Allium sativum) has been reported that have various beneficial effects including antistress, growth promotion, appetite stimulation, immunostimulation and antimicrobial properties in finfish and shrimp larviculture (Aly et al., 2010; Diab et al., 2008; Guo et al., 2011a; Millet et al., 2011; Nya and Austin, 2009; Peyghan et al., 2008; Sahu et al., 2007; Sasmal et al., 2005; Shalaby et al., 2006; Soko and Barker, 2005; Vaseeharan et al., 2011; Xie et al., 2009). The present study was carried out to understand the role of this plant extract on growth promotion, appetite stimulation, immunostimulation and biochemical constituents in body of Litopenaeus vannamei. In the present study, the dietary garlic extract supplementation led to enhanced trend on weight gain rate and SGR, but this increase was not significant. Khalil et al, (2001) mentioned that garlic contains allicin, which promotes the performance of the intestinal flora, thereby improving digestion and enhancing the utilization of energy, leading to growth improvement. Results of this study are comparable with the studies of Diab et al. (2002), Farahi et al. (2010), Nya and Austin (2009) and Shalaby et al. (2006), where significant growth rates were observed in treated fish over the controls. The crustacean hepatopancreas is a vital organ involved in excretion, molting, lipid and carbohydrate metabolism and diverse metabolic activities, including synthesis and secretion of digestive enzymes, absorption of nutrients, synthesis of plasma proteins such as hemocyanin and lipoproteins (Yepiz-Plascencia et al., 2000) and storage of energy reserves (Gibson and Barker, 1979). In crustacean, relative weight of hepatopancreas, which plays a key role in food assimilation (Dhall and Moriaty, 1984) and probably manifests the provision for energy utilization for growth and metabolism. Sagi and Ra’anan (1988) found that the relative size of hepatopancreas was highly correlated with the morphotypic stage of development and its relative energy expenditure in growth and sexual activity. The increase trend of HIS in experimental shrimp fed with garlic supplementation diets may be a consequence of the effect of chemical component in garlic extract on stimulation of the liver to produce and secrete the major pancreatic enzymes, enhance activities of pancreatic digestive enzymes and reserve energy in hepatopancerace (Platel and Srinivasan, 2004). In general, the growth curve of shrimp is similar to other crustaceans, i.e. a ladder style with much fast growth during each molting process. After molting, the size of crustacean rarely increases until the next molting (Dall et al., 1990).Wang et al. (2004) suggested that the growth of shrimp was correlated to feed, digestion and absorption of food, while the molting was related with the secretion of hormone. So there may be two distinct regulations of these two physical activities. In this study, higher MF resulted in faster growth in shrimps; while for shrimps with poor growth, the MF was relatively low. Thus, there was a clear correlation between growth and molting. It seems that garlic extract at level of 80 ppm can increase quality of basic diet.
Feed efficiency ratio (FER) and PER are used as quality indicators for shrimp diet and amino acid balance. So, these parameters are used to assess protein utilization and turnover. Feed quality, stocking density and water quality are the main factors affecting productivity for semi- intensive and intensive culture of penaeid shrimps (Cruz-Suárez et al., 1993). Wanapat et al. (2008) found significantly higher digestibility, absorption and retention of Nitrogen in garlic powder supplemented groups compared to control group. Our present finding also accords with these results that shrimp fed diet with garlic extract has higher Nitrogen utilization (PER,%) than control group.
The effects of garlic supplementation on protein metabolism have not been fully clarified. It is believed that garlic involved in hormonal secretion, may affect whole-body protein metabolism due to hormonal regulation by stimulating hormone secretion (noradrenaline and adrenaline), or it may affect protein metabolism by enhancing protein anabolism (Srivastava and Pathak, 2012). Oi et al. (2001) reported that protein anabolism occurs in rats fed the high protein diet supplemented with garlic. Garlic has depressed the hepatic activities of lipogenic and cholesterogenic enzymes such as malic enzyme, fatty acid synthase, glucose-6 phosphate dehydrogenase which are led to decreasing of lipid levels of body (Yeh and Liu, 2001) which is comparable with results obtained from current study. Recently some studies revealed that water-soluble organosulfur compounds, especially S-allyl cysteine (SAC) and diallyl-di-sulfide (DADS), are also potent inhibitors of cholesterol synthesis (Yeh and Liu, 2001; Gebhardt and Beck, 1996). The different composition and quantity of sulfur components of different garlic preparations used in various studies could be a reason for different findings. The mineral components are required for some important functions, including shell or bone building, blood clotting, muscle function and neuromascular transmission (Lovell, 1989; Coote et. al., 1996). Davis et al. 1992 reported that quality of feed can affect growth, survival and mineral component in body of P. vannamei. Increase of carcass ash content may be a result of food accessibility and mineral absorption by the animal (Tacon et al., 2002).
Crustaceans mainly rely on innate immunity to protect themselves against pathogen infections and other external factors that continually threaten their lives (Sarathi, et al., 2007).
Haemocytes play important roles in the immune response and based on the presence of cytoplasmic granules can be classified into three types: hyaline cells (HCs), semi-granular cells, and granular cells (GCs). HCs are involved in phagocytosis, whereas semi-granular cells and GCs are involved in the encapsulation and release of the prophenoloxidase (proPO) system (Jiravanichpaisal et al.,2006).
In the present study, the dietary garlic extract supplementation led to enhanced trend on THC. But, this increase trend was not significant. In many crustaceans, the sheet-like haematopoietic tissue covers the dorsal and dorsolateral sides of the stomach and is surrounded by connective tissue. Cells, believed to be haematopoietic cells, of different morphology are organized and densely packed in small lobules and some of these morphological cell types are also found in the interlobular spaces (Johansson et al., 2000). Molting, development of organs, reproductive status, nutritional condition and disease have been shown to influence haemocyte abundance ( Cheng and Chen, 2001). Tayag et al. (2010) expressed this fact that L. vannamei that received the extract via injection or immersion, all has been increased haemocyte count which suggests a proliferation of haemocyte in hematopoiesis. In the present study, shrimps which were fed by the garlic extract-containing diets increased haemocyte count.
The immune response is a physiological response and is normally regulated to maintain homeostasis. El-Desouky et al. (2012) expressed that small granular hemocytes were the most affected cell type by Z.officinalis and C. dactylon in Macrobrachium rosenbergii. Decreasing trend in semi granular and hyaline cells can be attributed to long term feeding period by diets containing garlic extract and its negative effect on hemostasis by storing immune factors (hemocytes) in hemopoetics tissues. It is notified that long term feeding by diets containing garlic extract may affect hemostasis of white leg shrimp and decreased hyaline and semi granular cells in the current study. Also, increase in granular cells and decrease in semi granular cells may be related to effect of garlic extract (Soltani, 2008). Granular cells contain antibacterial peptides that introduced as the first defense of host in innate immune system (Soltani, 2008).
In aquaculture, immunostimulants can stimulate the innate immune system by activation or increasing the activity of granulocytes and macrophages and increasing the number of phagocytes (Sakai, 1999; Yin et al., 2006, 2009). They can also increase protein synthesis to produce more of the molecules involved in innate metabolism system such as lysozyme, alkaline phosphatase and nitrogen monoxide (Xie et al., 2008; Rao et al., 2006; Liu et al., 2008). Blood serum protein is a biochemical system, precisely reflecting the condition of the organism and the changes happening to it under influence of internal and external factors. Total protein in serum was significantly high with shrimp fed on diet containing garlic extract which agrees with the results of Hussein et al (2001) who showed that serum total protein level was elevated in male albino rats after administration of garlic oil. Increase the total protein level in hyperlipidemic rats treated with Allium sativum oil could be attributed to the increase in the immunoglobulin level and total globulin concentration (Hussein, 1996). High serum protein levels have been reported due to improve liver and other organs functions which synthesized plasma protein (Metwally, 2009).
Conclusions
Finally, based on the results obtained from this study, garlic extract may be applied as a food supplementation in shrimp aquaculture, but using this extract should be done after supplementary examination with other levels of extract and at different stages of shrimp life.
By Samadi, Leila; Zanguee, Nasim; Mousavi, Seyed Mohammad; Zakeri, Mohammad

 Tiếng Việt
Tiếng Việt